If it feels like there is a never-ending amount of infection control information and requirements to be aware of, you’re not alone. That’s why we’re back with more additions to our Ftag of the Week series for F880 Infection Prevention and Control. This time, we’re discussing the brand-new requirements for using Enhanced Barrier Precautions in nursing homes, guidance which was added to this regulation on March 20, 2024. Not only was it just added, but it’s also effective April 1, 2024, so if you aren’t already using this CDC-recommended practice, you’ve got a short timeframe to figure out what to do before your next survey.
On March 20, 2024, the Centers for Medicare and Medicaid Services (CMS) released a new QSO Memo, “Enhanced Barrier Precautions in Nursing Homes.” Review the QSO Memo and its guidance in this accompanying CMSCG Blog post. In this post, we’ll review the new guidance included under F880 since the regulatory language hasn’t been updated for these requirements.
Enhanced Barrier Precautions – Defined
EBP is defined as an infection control intervention that is designed to reduce the transmission of multi-drug resistant organisms (MDROs) by employing targeted gown and glove use during high contact resident care activities which provide opportunities for the transfer or MDROs to staff hands and/or clothing.
EBP is indicated for residents exhibiting any of the following:
- Infection or colonization with a CDC-targeted MDRO when use of contact precautions does not apply
- Wounds and/or indwelling medical devices even if the resident is not known to be infected or colonized with an MDRO. Wounds, for the purpose of these requirements, are chronic wounds, such as pressure ulcers or unhealed surgical wounds, not wounds such as skin breaks/tears which are covered with a dressing and are shorter-lasting. Indwelling medical devices include urinary catheters, trachs, central lines and feeding tubes.
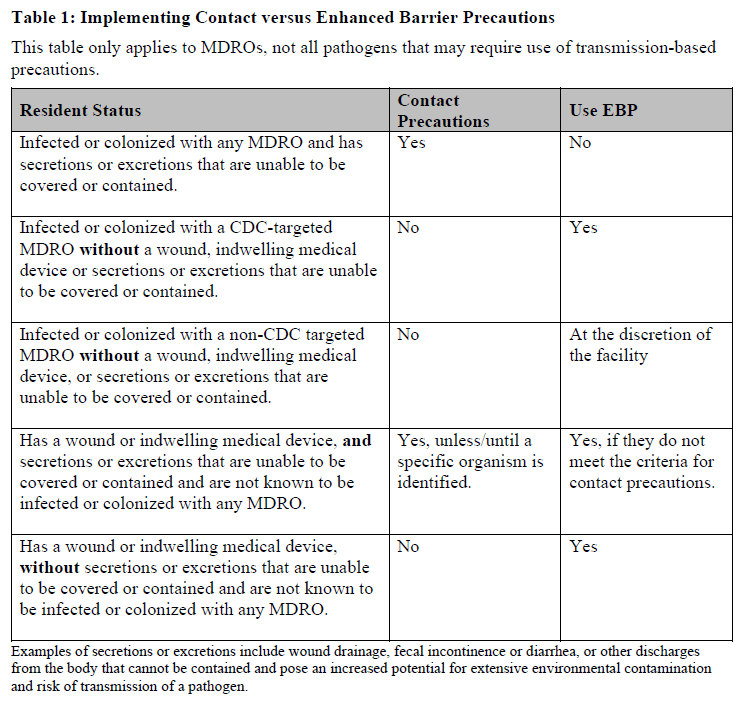
There’s a table included in the guidance which defines when Contact Precautions should be implemented versus when Enhanced Barrier Precautions should be used.
Please refer to this table when you are developing your protocols and providing staff education.
High-Contact Resident Care Activities
Use of EBP is indicated for residents who meet the above criteria during the following activities, which are considered “high contact” activities:
- Dressing
- Transferring
- Changing linens
- Bathing/Showering
- Providing hygiene
- Changing briefs
- Toileting assistance
- Care or use of devices (central line, urinary catheter, feeding tube, tracheostomy, ventilator)
- Caring for a wound (any skin opening requiring a dressing)
Gown and Glove Use
It’s important to note that the new guidance states that in general, the use of a gown and gloves would not be recommended in common areas since contact with residents would be of shorter duration. So that applies to areas such as dining rooms or activities rooms. That recommendation does not apply outside of the resident’s room in:
- shared/common areas such as a shower/tub room when performing transfers or providing bathing assistance
- when staff are working in a therapy gym and anticipate close physical contact while assisting a resident with transfers and/or mobility
Remember, since EBP are used only during high-contact activities, use of PPE would not need to be donned prior to entering a resident’s room. The guidance states the following example: “staff entering the resident’s room to answer a call light, converse with a resident or provide medications who do not engage in high-contact resident care activity would likely not need to employ EBP while interacting with the resident.”
Duration of EBP
EBP is considered to be less restrictive than the use of Contact Precautions, since the residents are not restricted to their rooms or prevented from socializing/ attending activities with other residents to reduce transmission risk. EBP must remain in place for the duration of the resident’s stay until either the wound resolves or the use of the indwelling medical device is discontinued.
Other Guidance to be Aware of
The QSO Memo also includes some other guidance worth reviewing. The Memo indicates that providers have “discretion” on how to communicate the need for EBP for residents requiring it, noting that CMS support “creative” ways to notify staff while maintaining a homelike environment. What matters is that staff know which residents require use of EBP prior to high-contact activities. It remains to be seen, though, when this requirement is in effect how well this liberal communication of PPE requirements is followed by staff before CMS may consider requiring specific signage.
CMSCG will post updated survey resources once CMS releases them. Need help implementing Enhanced Barrier Precautions in your facility? CMS Compliance Group’s Clinical Consultants have the experience and expertise to assist you with development of associated procedures or implementation challenges. Contact us today to learn more.