On November 12, 2021, the Centers for Medicare & Medicaid (CMS) released a QSO Memo, “Changes to COVID-19 Survey Activities and Increased Oversight in Nursing Homes” (QSO-22-02-ALL). The Memo provides information on changes that CMS is making to help State Survey Agencies (SAs) get caught up with a significant backlog of recertification and complaint surveys. Coupled with these survey changes is also the announcement that nursing homes will be subject to increased oversight from CMS.
How Will the Survey Changes Impact Nursing Homes?
To truly understand the impact of the changes that CMS is making to survey activities, we need to discuss how much of a survey backlog there is due to limited survey & certification activities during the COVID-19 Public Health Emergency (PHE). According to available CASPER data, as of November 14, 2021, 33.5% of nursing homes in the country have not had a standard recertification survey for 24 months or more. Given that the maximum time allowed between these surveys is 15 months, the State Survey Agencies have a lot of catch-up work to try to accomplish after focusing primarily on conducting Focused Infection Control (FIC) Surveys and conducting limited complaint investigations. Thus, not only are there over 5,000 nursing homes that haven’t been surveyed in over two years, there are also tons of open complaints that surveyors need to address.
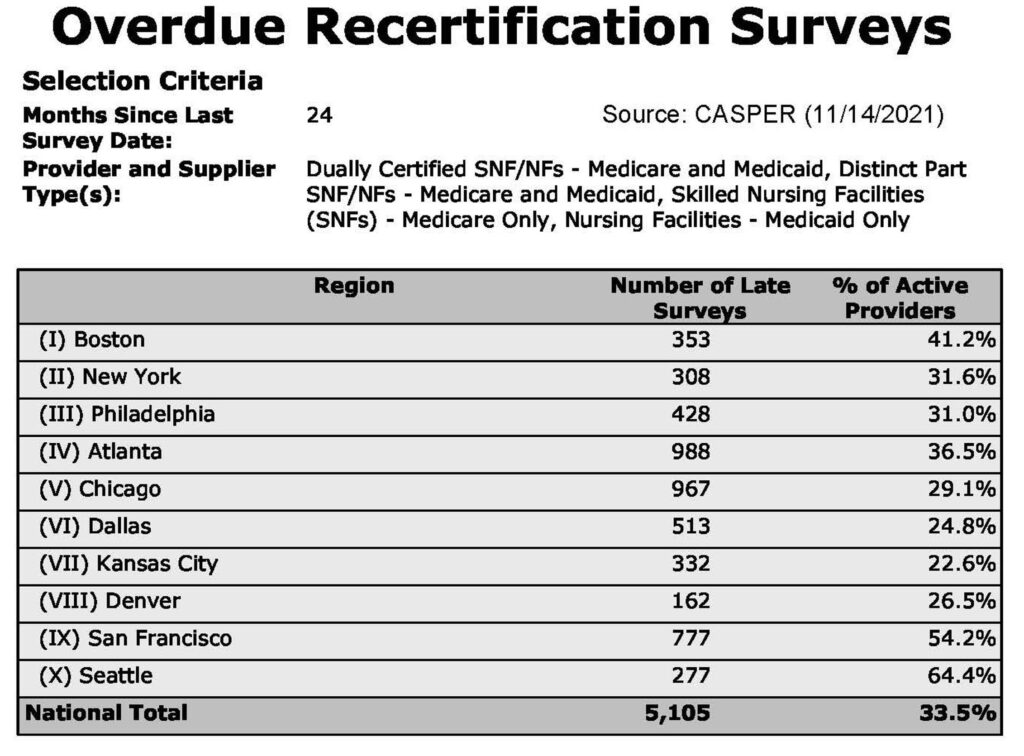
As of November 14, 2021, 33.5% of nursing homes in the country have not had a standard recertification survey for 24 months or more.
Source: CASPER (11/14/2021)
So, in order to help State Agencies do a little catch-up, CMS is making numerous changes to survey activities. These include:
- Limiting the number of FIC Surveys
- Resuming standard recertification surveys
- Providing flexibilities regarding complaints and Facility-Reported Incidents (FRIs)
- Working with States to adjust timeframes for clearing survey backlogs
- Providing temporary flexibilities and guidance to help surveyors work through the backlog
Changes to Focused Infection Control Surveys
Per the CMS QSO-20-31-ALL Memo, CMS had mandated that FIC surveys were conducted within 3-5 days when a nursing home was having a COVID-19 outbreak (3+ new confirmed cases or 1 confirmed resident case in a facility that had previously been COVID-19 free). That requirement is now removed, which hopefully means that the days of facilities having half a dozen or more infection control surveys are behind us. The Memo states that if COVID-19 infection concerns arise, that a State may still conduct a FICS.
However, SAs are still required to perform annual FIC surveys of 20% of nursing homes in the State, which had been previously announced. CMS has instructed the States to prioritize these surveys at facilities where new COVID-19 cases are being reported and the facility has low vaccination rates. Other things to know about the annual FICS:
- The survey must be stand-alone and not included in a recertification survey
- The FICS can be combined with a complaint survey. This has changed from the prior guidance where, in order to count towards the annual 20%, the survey had to be independent.
States are incentivized to complete these survey activities within expected time frames and in line with other expectations or could forfeit some of their CARES Act allocation each year. So, providers with low vaccination rates that have new COVID-19 cases are the most likely to see one of these focused surveys, but in States where vaccinations are mandatory, the State will still be obligated to conduct some of these surveys. Remember, the FIC survey can capture many concerns that surveyors identify – not just how the facility is handling COVID-19-related infection control. Multiple Infection Control deficiencies at F880 cited during a FICS have nothing to do with COVID-19 management.
In the next part of this post on the CMSCG Blog, we will look at important changes to recertification surveys. Stay tuned. If you are preparing for survey and would like assistance, please contact us to learn more about our survey preparation services for nursing homes. To learn more about CMS Compliance Group, Inc., please visit our website.